Which of the Following Is Used to Determine Stoichiometric Factors
Perform stoichiometric calculations involving mass moles and solution molarity. He Kjeldahl analysis is used to determine the protein content of foods.

6 5 Mole Mass And Mass Mass Problems The Basics Of General Organic And Biological Chemistry
Your email address will not be published.

. NH 3g BF 3g. Stoichiometry Stoichiometric Calculations Starting with 10. The flow chart below summarizes the process.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. 471molC4H1013molO22molC4H1030615molO2 The answer should have three significant figures so round to 306molO2. D 6 mol H 2 O4 mol NH 3.
The food analyzed is heated. 542 g 1600 gmol 339 mol 338 1 2 2. Use the stoichiometric factor 13molO22molC4H10 to calculate moles of O2 from moles of C4H10.
Which of the following is used to determine stoichiometric factors. Chemical formulas provide the identities of the reactants and products involved in the. C 4 mol H 2 O6 mol NH 3.
The Haber process for producing ammonia commercially is represented by the equation N2 3H2 ---- 2NH3. The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. Leave a Reply Cancel reply.
Which of the following stoichiometric factors would be used to convert from moles of NH 3 to moles of H 2 O for the reaction. While adding a step to a stoichiometric problem this doesnt make the problem more difficult as long as you remember the coefficients in a balanced chemical equation relate the. Required fields are marked.
Stoichiometry helps us determine how much substance is needed or is present. The number of significant figures of any measured quantities in the problem. The easiest way to do stoichiometric calculations involves using conversion factors.
Which of the following is used to determine stoichiometric factors. A conversion factor is a ratio or fraction which represents the relationship between two different units. Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth.
TF Stoichiometry problems can be solved with conversion factors created from mole ratios molar masses and Avogadros nunber. We can write a mole ratio for a pair of substances by looking at the coefficients in front of each species in the balanced chemical equation. B 4 mol NH 3 6 mol H 2 O.
Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. How many moles of O2 are required to react with 30 moles of P4. Stoichiometry measures these quantitative relationships and is used to determine the amount of products and reactants that are produced or needed in a given reaction.
Mole ratio of any two substances in the reaction. The molecular formula of this compound is C4H6O4. Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth.
For example in the reaction. Convert the units of the given substance A to moles. There are four steps in solving a stoichiometry problem.
Write the balanced chemical equation. What are the possible stoichiometric factors for CH42O2--- CO22H2O. Reactants and Products mass.
Determine the stoichiometric factor in the following balanced chemical equation. Here are some examples of conversion factors. A balanced chemical equation allows one to determine the.
A balanced chemical equation provides a great deal of information in a very succinct format. QUESTION 1 The following data may be used for the questions that follow. The conversion factor used to convert from grams to moles is _____.
A 5 mol O 2 4 mol NH 3. The number of significant figures in the mole ratio used to solve the problem. Convert moles of the wanted substance to the desired units.
10 grams of glucose C 6H 12O 6 react in a combustion reaction. Things that can be measured are. First I had to find the molar mass of the empirical number by multiplying each value from the mole ratio by the elements molar mass.
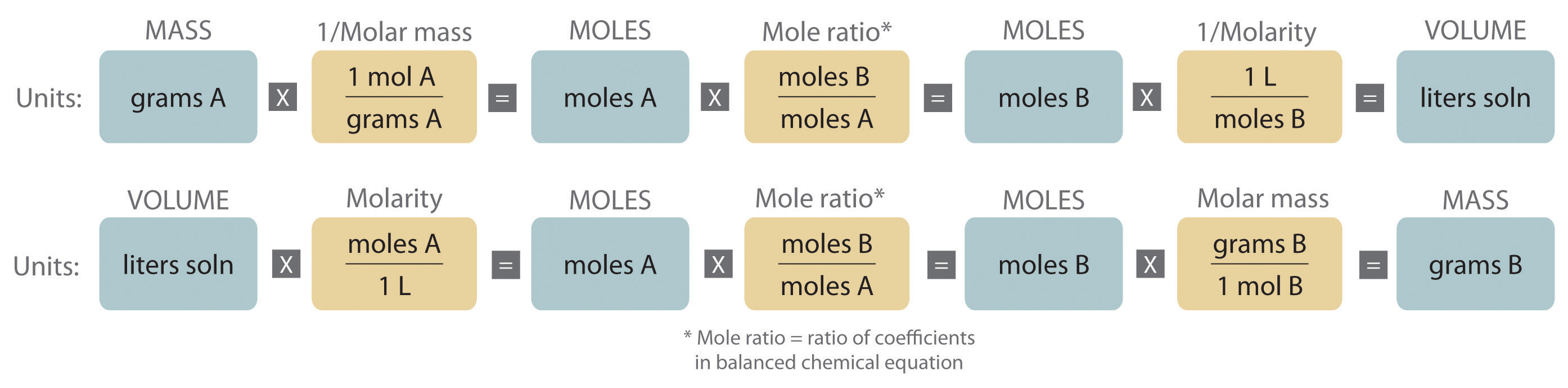
Stoichiometric Factors In a laboratory experiment amounts of substances are usually measured on a scale mass or with some type of measuring glassware volume. The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. In the example above reaction stoichiometry measures the.
Almost all stoichiometric problems can be solved in just four simple steps. G of C 6H 12O 6 we calculate the moles of C 6H 12O 6 use the coefficients to find the moles of H 2O CO 2 and then turn the moles to grams C 6H 12O 6s 6 O 2g 6 CO 2g 6 H 2O l 10g. 51 g 1008 gmol 514 mol 338 152 2 3.
Balanced equations and mole ratios. Stoichiometric coefficient or stoichiometric number is the number of molecules that participate in the reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry.
Convert units of a given substance to moles. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. Inverted molar mass In the following stoichiometry problem how many moles of oxygen are produced when 30 mol of KClO3 decompose completely.
The number of significant figures in an answer to a stoichiometry problem is determined only by a. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products. A common type of stoichiometric relationship is the mole ratio which relates the amounts in moles of any two substances in a chemical reaction.
A conversion factor is ALWAYS equal to 1. The number of decimal places in the molar masses of substances in the chemical equation. Use the mole ratio to calculate the moles of wanted substance B.
Is a 11 rario correct. 4NH 3 5O 2 4NO 6H 2 O. Determine the stoichiometric factor in the following balanced chemical equation.
Is a 11 rario correct. Applying Conversion Factors to Stoichiometry Now youre ready to use what you know about conversion factors to solve some stoichiometric problems in chemistry.
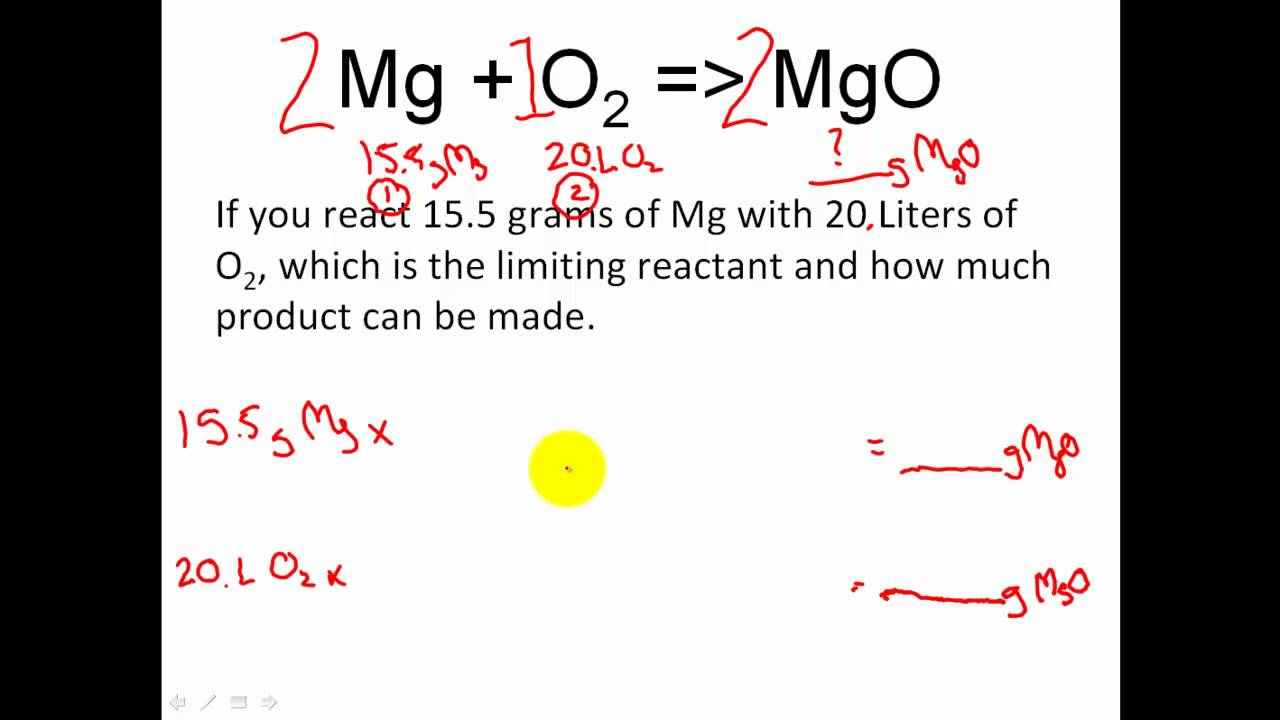
Reaction Stoichiometry Boundless Chemistry

Stoichiometry And Stoichiometric Calculations Concepts Videos Example

Green Policy Sustainability And Safety The Main Purpose Of The Green Metrics Is To Obtain Clear Simple And Fast Green Chemistry Organic Synthesis Chemistry

Reaction Stoichiometry Boundless Chemistry

Green Manufacturing Green Manufacturing Is A Method Of Manufacturing That Reduces Waste And Pollution Green Ma Green Manufacturing Green Chemistry Chemistry

Reaction Stoichiometry Boundless Chemistry

7 4 Reaction Stoichiometry Introductory Chemistry

Introduction To Limiting Reactant And Excess Reactant Science Sciencewithtylerdewitt Tylerdewitt Tu Chemistry Help Apologia Chemistry High School Chemistry

Lesson Explainer Reaction Masses Nagwa
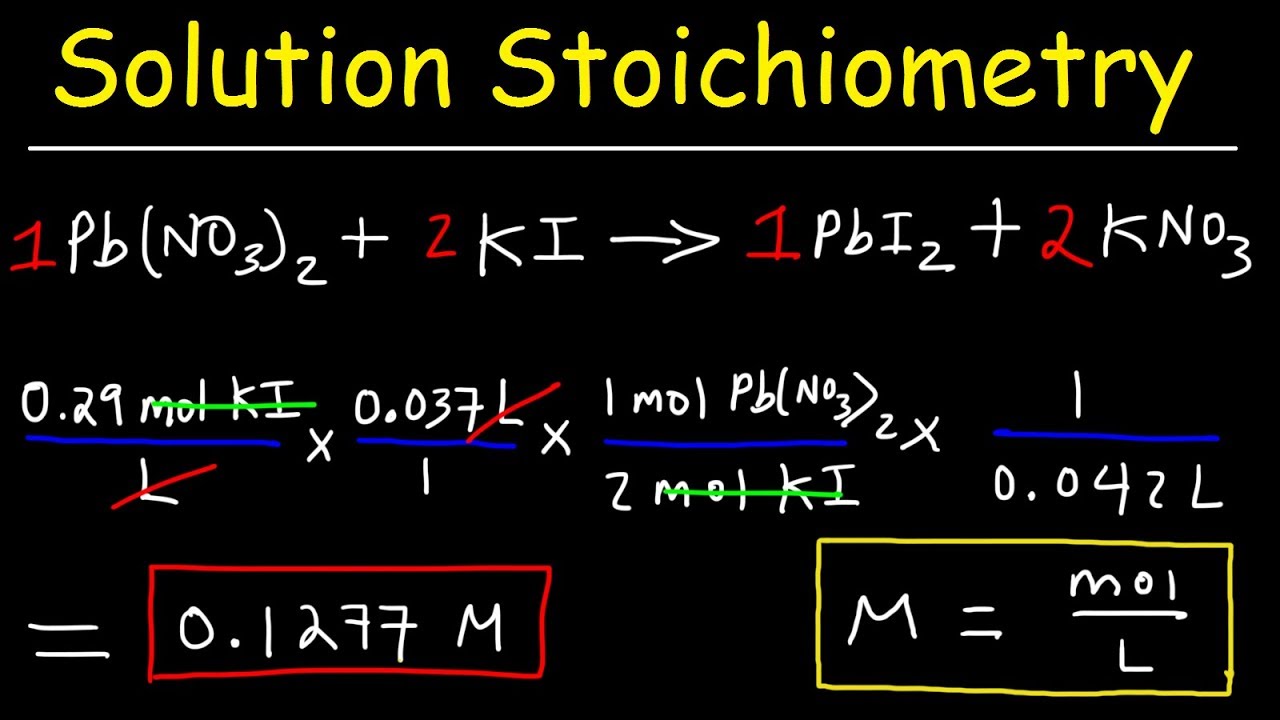
Solution Stoichiometry Finding Molarity Mass Volume Youtube

Stoichiometry Chemistry Activities

4 6 Solution Stoichiometry And Chemical Analysis Chemistry Libretexts
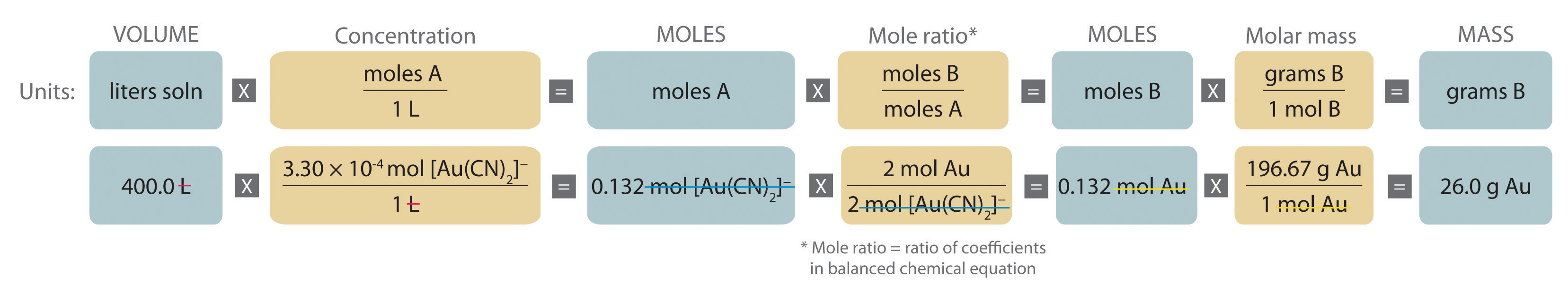
Stoichiometry Of Reactions In Solution
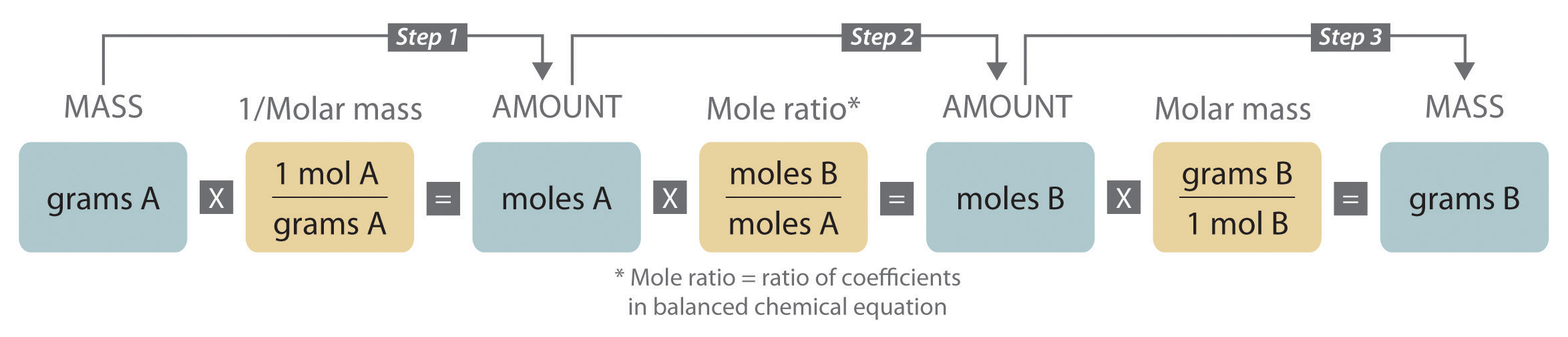
3 6 Reaction Stoichiometry Chemistry Libretexts
Stoichiometry Of Reactions In Solution

Chapter 9 Stoichiometry Pages Intro To Stoichiometry All Stoichiometric Calculations Start With A To Solve You Ppt Download


Comments
Post a Comment